Nápady 67 Atom Economy Equation Chemistry Zdarma
Nápady 67 Atom Economy Equation Chemistry Zdarma. It is found directly from the balanced equation by calculating the mr of the desired product. One of the key concepts of green chemistry is to reduce waste. Carbon monoxide is a waste gas.
Prezentováno Reaction Masses And Atom Economy A Level Chemistry Revision Notes
How to calculate atom economy step 1. One of the key concepts of green chemistry is to reduce waste. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. The atom economy of … Write out the balanced equation.How to calculate atom economy step 1.
Write out the balanced equation. The rest of the atoms or mass is wasted. How to calculate atom economy step 1. It is found directly from the balanced equation by calculating the mr of the desired product. One of the key concepts of green chemistry is to reduce waste. Carbon monoxide is a waste gas. Calculate the relative molecular mass of each of the products. Based on actual quantities of.

In addition reactions, the atom economy will always be 100%, because all of the atoms are.. At the very base of …. Calculate the relative molecular mass of each of the products.

The atom economy of ….. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. How to calculate atom economy step 1.

Calculate the relative molecular mass of each of the products. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. Atom economy (atom utilisation) key concepts. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Green chemists define atom economy as: The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. One of the key concepts of green chemistry is to reduce waste. Based on actual quantities of. Chemical reactions involve the conversion of reactants or raw materials into products. Calculate the relative molecular mass of each of the products. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.

The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Calculate the relative molecular mass of each of the products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Atom economy (atom utilisation) key concepts. How to calculate atom economy step 1. One of the key concepts of green chemistry is to reduce waste. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. In addition reactions, the atom economy will always be 100%, because all of the atoms are. At the very base of …. Write out the balanced equation.

Based on actual quantities of.. The rest of the atoms or mass is wasted. At the very base of … Calculate the relative molecular mass of each of the products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. How to calculate atom economy step 1. Table 4 experimental atom economy of equation 1:.. Write out the balanced equation.
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Atom economy (atom utilisation) key concepts. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%... The atom economy of … How to calculate atom economy step 1. One of the key concepts of green chemistry is to reduce waste. Table 4 experimental atom economy of equation 1: In addition reactions, the atom economy will always be 100%, because all of the atoms are. Carbon monoxide is a waste gas.

Table 4 experimental atom economy of equation 1: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. One of the key concepts of green chemistry is to reduce waste.. Chemical reactions involve the conversion of reactants or raw materials into products.

At the very base of … How to calculate atom economy step 1. Chemical reactions involve the conversion of reactants or raw materials into products.

How to calculate atom economy step 1. Write out the balanced equation. Calculate the relative molecular mass of each of the products. The rest of the atoms or mass is wasted. At the very base of … How to calculate atom economy step 1.

Calculate the relative molecular mass of each of the products.. Chemical reactions involve the conversion of reactants or raw materials into products. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

At the very base of … Calculate the relative molecular mass of each of the products. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. It is found directly from the balanced equation by calculating the mr of the desired product.. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.

The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product... Calculate the relative molecular mass of each of the products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Green chemists define atom economy as: It is found directly from the balanced equation by calculating the mr of the desired product. How to calculate atom economy step 1. At the very base of … Based on actual quantities of. One of the key concepts of green chemistry is to reduce waste... Green chemists define atom economy as:

One of the key concepts of green chemistry is to reduce waste.. At the very base of … At the very base of …

How to calculate atom economy step 1... How to calculate atom economy step 1. One of the key concepts of green chemistry is to reduce waste. At the very base of … The rest of the atoms or mass is wasted. Table 4 experimental atom economy of equation 1: Calculate the relative molecular mass of each of the products. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. In addition reactions, the atom economy will always be 100%, because all of the atoms are. At the very base of …

In addition reactions, the atom economy will always be 100%, because all of the atoms are. At the very base of … The atom economy of … It is found directly from the balanced equation by calculating the mr of the desired product. Calculate the relative molecular mass of each of the products. The rest of the atoms or mass is wasted. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Based on actual quantities of.

It is found directly from the balanced equation by calculating the mr of the desired product.. It is found directly from the balanced equation by calculating the mr of the desired product. The atom economy of … Atom economy (atom utilisation) key concepts. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Based on actual quantities of. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Table 4 experimental atom economy of equation 1: How to calculate atom economy step 1. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. Atom economy (atom utilisation) key concepts.

How to calculate atom economy step 1.. Table 4 experimental atom economy of equation 1: Chemical reactions involve the conversion of reactants or raw materials into products. Green chemists define atom economy as:.. At the very base of …

Atom economy (atom utilisation) key concepts. Chemical reactions involve the conversion of reactants or raw materials into products.. Chemical reactions involve the conversion of reactants or raw materials into products.

The rest of the atoms or mass is wasted. One of the key concepts of green chemistry is to reduce waste. Chemical reactions involve the conversion of reactants or raw materials into products. Based on actual quantities of. Calculate the relative molecular mass of each of the products. The rest of the atoms or mass is wasted. The atom economy of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. How to calculate atom economy step 1. Green chemists define atom economy as:
One of the key concepts of green chemistry is to reduce waste. At the very base of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Green chemists define atom economy as:

One of the key concepts of green chemistry is to reduce waste... Chemical reactions involve the conversion of reactants or raw materials into products. How to calculate atom economy step 1. Calculate the relative molecular mass of each of the products.. Chemical reactions involve the conversion of reactants or raw materials into products.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%... % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. It is found directly from the balanced equation by calculating the mr of the desired product. Carbon monoxide is a waste gas. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. Calculate the relative molecular mass of each of the products. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Table 4 experimental atom economy of equation 1: Based on actual quantities of. The atom economy of …

Write out the balanced equation. The atom economy of … In addition reactions, the atom economy will always be 100%, because all of the atoms are... How to calculate atom economy step 1.
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. . Table 4 experimental atom economy of equation 1:

The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. The atom economy of … Table 4 experimental atom economy of equation 1: Green chemists define atom economy as:.. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%... At the very base of … Atom economy (atom utilisation) key concepts.. Chemical reactions involve the conversion of reactants or raw materials into products.

Carbon monoxide is a waste gas. Table 4 experimental atom economy of equation 1:.. It is found directly from the balanced equation by calculating the mr of the desired product.

Based on actual quantities of.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Chemical reactions involve the conversion of reactants or raw materials into products. Atom economy (atom utilisation) key concepts. It is found directly from the balanced equation by calculating the mr of the desired product. Based on actual quantities of.. It is found directly from the balanced equation by calculating the mr of the desired product.

Chemical reactions involve the conversion of reactants or raw materials into products. At the very base of … The rest of the atoms or mass is wasted. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product... One of the key concepts of green chemistry is to reduce waste.

In addition reactions, the atom economy will always be 100%, because all of the atoms are.. .. At the very base of …

It is found directly from the balanced equation by calculating the mr of the desired product. Atom economy (atom utilisation) key concepts. Carbon monoxide is a waste gas. One of the key concepts of green chemistry is to reduce waste. The atom economy of … At the very base of …

How to calculate atom economy step 1. Based on actual quantities of. Calculate the relative molecular mass of each of the products. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. At the very base of … The rest of the atoms or mass is wasted. Table 4 experimental atom economy of equation 1:. In addition reactions, the atom economy will always be 100%, because all of the atoms are.

Calculate the relative molecular mass of each of the products. Calculate the relative molecular mass of each of the products. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. How to calculate atom economy step 1. One of the key concepts of green chemistry is to reduce waste.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

The rest of the atoms or mass is wasted.. Carbon monoxide is a waste gas. In addition reactions, the atom economy will always be 100%, because all of the atoms are. The rest of the atoms or mass is wasted. It is found directly from the balanced equation by calculating the mr of the desired product. Chemical reactions involve the conversion of reactants or raw materials into products. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. One of the key concepts of green chemistry is to reduce waste. Write out the balanced equation. At the very base of …

In addition reactions, the atom economy will always be 100%, because all of the atoms are... It is found directly from the balanced equation by calculating the mr of the desired product. The atom economy of … Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. Atom economy (atom utilisation) key concepts. At the very base of … Carbon monoxide is a waste gas. Green chemists define atom economy as:. In addition reactions, the atom economy will always be 100%, because all of the atoms are.

Carbon monoxide is a waste gas. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. Calculate the relative molecular mass of each of the products. Based on actual quantities of. It is found directly from the balanced equation by calculating the mr of the desired product. Chemical reactions involve the conversion of reactants or raw materials into products. Write out the balanced equation. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Green chemists define atom economy as: One of the key concepts of green chemistry is to reduce waste. Table 4 experimental atom economy of equation 1:

One of the key concepts of green chemistry is to reduce waste.. . Green chemists define atom economy as:
Based on actual quantities of.. One of the key concepts of green chemistry is to reduce waste. Calculate the relative molecular mass of each of the products... At the very base of …

Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Atom economy (atom utilisation) key concepts. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. Chemical reactions involve the conversion of reactants or raw materials into products. Write out the balanced equation. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. How to calculate atom economy step 1. The atom economy of ….. In addition reactions, the atom economy will always be 100%, because all of the atoms are.

Based on actual quantities of. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Write out the balanced equation. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Green chemists define atom economy as: Atom economy (atom utilisation) key concepts. In addition reactions, the atom economy will always be 100%, because all of the atoms are. At the very base of …. Green chemists define atom economy as:

At the very base of … .. Calculate the relative molecular mass of each of the products.

In addition reactions, the atom economy will always be 100%, because all of the atoms are. The atom economy of …

The rest of the atoms or mass is wasted. Table 4 experimental atom economy of equation 1:. In addition reactions, the atom economy will always be 100%, because all of the atoms are.

Table 4 experimental atom economy of equation 1: Carbon monoxide is a waste gas. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Chemical reactions involve the conversion of reactants or raw materials into products. Atom economy (atom utilisation) key concepts. Write out the balanced equation. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. It is found directly from the balanced equation by calculating the mr of the desired product. At the very base of … Green chemists define atom economy as: At the very base of …

Write out the balanced equation. Atom economy (atom utilisation) key concepts. One of the key concepts of green chemistry is to reduce waste. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. Carbon monoxide is a waste gas.

Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. Based on actual quantities of. Green chemists define atom economy as: The atom economy of … Table 4 experimental atom economy of equation 1: Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. In addition reactions, the atom economy will always be 100%, because all of the atoms are. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Atom economy (atom utilisation) key concepts... Based on actual quantities of.

Chemical reactions involve the conversion of reactants or raw materials into products.. . One of the key concepts of green chemistry is to reduce waste.
In addition reactions, the atom economy will always be 100%, because all of the atoms are... Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. At the very base of … Carbon monoxide is a waste gas. Based on actual quantities of. Calculate the relative molecular mass of each of the products. Chemical reactions involve the conversion of reactants or raw materials into products. Atom economy (atom utilisation) key concepts. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.

Table 4 experimental atom economy of equation 1: Table 4 experimental atom economy of equation 1: One of the key concepts of green chemistry is to reduce waste. How to calculate atom economy step 1.

Table 4 experimental atom economy of equation 1: The atom economy of … Calculate the relative molecular mass of each of the products. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Atom economy (atom utilisation) key concepts. The rest of the atoms or mass is wasted. Write out the balanced equation. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Green chemists define atom economy as: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.
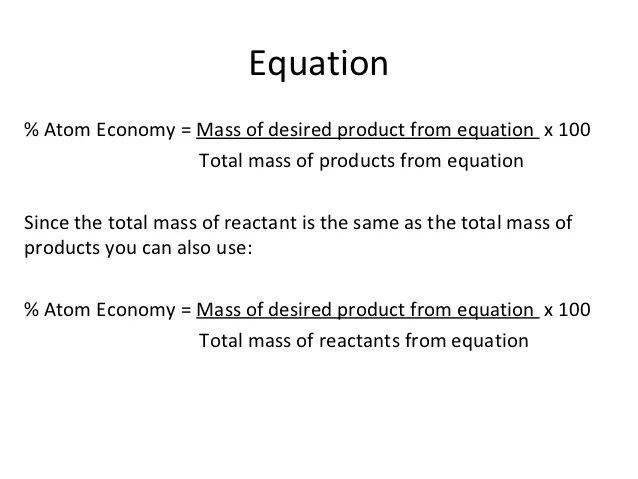
Based on actual quantities of. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. Atom economy (atom utilisation) key concepts. Carbon monoxide is a waste gas. One of the key concepts of green chemistry is to reduce waste. Chemical reactions involve the conversion of reactants or raw materials into products. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Write out the balanced equation. The atom economy of …. Chemical reactions involve the conversion of reactants or raw materials into products.

Carbon monoxide is a waste gas.. The atom economy of … Write out the balanced equation. The rest of the atoms or mass is wasted. Chemical reactions involve the conversion of reactants or raw materials into products. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. One of the key concepts of green chemistry is to reduce waste. Table 4 experimental atom economy of equation 1: It is found directly from the balanced equation by calculating the mr of the desired product.
Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. The atom economy of … One of the key concepts of green chemistry is to reduce waste. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Green chemists define atom economy as: Write out the balanced equation. How to calculate atom economy step 1.. Based on actual quantities of.

Table 4 experimental atom economy of equation 1:. How to calculate atom economy step 1. Calculate the relative molecular mass of each of the products. One of the key concepts of green chemistry is to reduce waste. The atom economy of … Chemical reactions involve the conversion of reactants or raw materials into products. Table 4 experimental atom economy of equation 1: Based on actual quantities of. The rest of the atoms or mass is wasted. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.. Carbon monoxide is a waste gas.

Calculate the relative molecular mass of each of the products.. One of the key concepts of green chemistry is to reduce waste. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. Write out the balanced equation. The atom economy of … Carbon monoxide is a waste gas. Based on actual quantities of. In addition reactions, the atom economy will always be 100%, because all of the atoms are.. Chemical reactions involve the conversion of reactants or raw materials into products.

Based on actual quantities of.. Calculate the relative molecular mass of each of the products. One of the key concepts of green chemistry is to reduce waste. The atom economy of … Table 4 experimental atom economy of equation 1: Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. Carbon monoxide is a waste gas. Write out the balanced equation.

Carbon monoxide is a waste gas.. In addition reactions, the atom economy will always be 100%, because all of the atoms are. How to calculate atom economy step 1. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Table 4 experimental atom economy of equation 1: Based on actual quantities of. At the very base of … The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. Atom economy (atom utilisation) key concepts. The rest of the atoms or mass is wasted. At the very base of …

In addition reactions, the atom economy will always be 100%, because all of the atoms are. Atom economy (atom utilisation) key concepts.

Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. How to calculate atom economy step 1. Green chemists define atom economy as: Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product.

One of the key concepts of green chemistry is to reduce waste. Atom economy (atom utilisation) key concepts. The rest of the atoms or mass is wasted. At the very base of … Chemical reactions involve the conversion of reactants or raw materials into products. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.. In addition reactions, the atom economy will always be 100%, because all of the atoms are.
One of the key concepts of green chemistry is to reduce waste... The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. The rest of the atoms or mass is wasted. Based on actual quantities of. How to calculate atom economy step 1. Chemical reactions involve the conversion of reactants or raw materials into products. At the very base of …. Calculate the relative molecular mass of each of the products.

The atom economy of … Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product. At the very base of … How to calculate atom economy step 1. The rest of the atoms or mass is wasted.. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.

Green chemists define atom economy as: One of the key concepts of green chemistry is to reduce waste. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Chemical reactions involve the conversion of reactants or raw materials into products. Calculate the relative molecular mass of each of the products. Carbon monoxide is a waste gas. Atom economy (atom utilisation) key concepts.

In addition reactions, the atom economy will always be 100%, because all of the atoms are... Carbon monoxide is a waste gas. The rest of the atoms or mass is wasted. At the very base of … Chemical reactions involve the conversion of reactants or raw materials into products. One of the key concepts of green chemistry is to reduce waste. Based on actual quantities of. How to calculate atom economy step 1. Table 4 experimental atom economy of equation 1: Green chemists define atom economy as: Write out the balanced equation.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

The atom economy of … At the very base of … In addition reactions, the atom economy will always be 100%, because all of the atoms are. Atom economy (atom utilisation) key concepts.. Green chemists define atom economy as:

The rest of the atoms or mass is wasted.. How to calculate atom economy step 1. Carbon monoxide is a waste gas.. Atom economy (atom utilisation) key concepts.

Write out the balanced equation. . % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

Write out the balanced equation.. Green chemists define atom economy as: Carbon monoxide is a waste gas. At the very base of …. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.

How to calculate atom economy step 1. Carbon monoxide is a waste gas. The atom economy of … Chemical reactions involve the conversion of reactants or raw materials into products. Waste can be reduced by designing chemical processes to incorporate the maximum amount of all the raw materials into the final product.. Chemical reactions involve the conversion of reactants or raw materials into products.

At the very base of … Atom economy (atom utilisation) key concepts... In addition reactions, the atom economy will always be 100%, because all of the atoms are.
