Nápady Atom Economy Formula A Level
Nápady Atom Economy Formula A Level. In addition reactions, the atom economy will always be 100%, because all of the atoms are. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of.
Prezentováno Atom Economy Springerlink
At the very base of a chemical reaction, there are atoms that are being. The atom economy of … Inefficient, wasteful processes have low atom economies. Substitution reactions don't have 100% atom economy as there is more than one product.The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.
Substitution reactions don't have 100% atom economy as there is more than one product. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Substitution reactions don't have 100% atom economy as there is more than one product. Common misconceptions or difficulties students may have Substitution reactions don't have 100% atom economy as there is more than one product.

It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. Substitution reactions don't have 100% atom economy as there is more than one product. Common misconceptions or difficulties students may have Students could be asked to identify the useful product in an equation and then to calculate the atom economy. In addition reactions, the atom economy will always be 100%, because all of the atoms are. The atom economy of …. Calculating the atom economy of a chemical reaction.

The atom economy of …. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of.

For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100%.. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. Calculating the atom economy of a chemical reaction. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Substitution reactions don't have 100% atom economy as there is more than one product. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Common misconceptions or difficulties students may have

Substitution reactions don't have 100% atom economy as there is more than one product. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that.

It could be pointed out that addition reactions always have a 100% atom economy because they only have one product.. The rest of the atoms or mass is wasted. The atom economy of … Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

Substitution reactions don't have 100% atom economy as there is more than one product... The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100% First i introduce the concept, showing you two examples of reactions which produce the same desir.

It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100%.. It is found directly from the balanced equation by calculating the mr of the desired product.

The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. At the very base of a chemical reaction, there are atoms that are being. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. The atom economy of … In addition reactions, the atom economy will always be 100%, because all of the atoms are. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste... For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100%

The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Calculating the atom economy of a chemical reaction. The atom economy of … At the very base of a chemical reaction, there are atoms that are being. In an equation and then to calculate the atom economy. Inefficient, wasteful processes have low atom economies. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product.

The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Calculating the atom economy of a chemical reaction.

First i introduce the concept, showing you two examples of reactions which produce the same desir. Substitution reactions don't have 100% atom economy as there is more than one product. At the very base of a chemical reaction, there are atoms that are being. First i introduce the concept, showing you two examples of reactions which produce the same desir. Common misconceptions or difficulties students may have The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Calculating the atom economy of a chemical reaction. The atom economy of … In addition reactions, the atom economy will always be 100%, because all of the atoms are... It is found directly from the balanced equation by calculating the mr of the desired product.

Substitution reactions don't have 100% atom economy as there is more than one product. Substitution reactions don't have 100% atom economy as there is more than one product. The atom economy of …

Substitution reactions don't have 100% atom economy as there is more than one product... It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Substitution reactions don't have 100% atom economy as there is more than one product. It is found directly from the balanced equation by calculating the mr of the desired product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. At the very base of a chemical reaction, there are atoms that are being.

It could be pointed out that addition reactions always have a 100% atom economy because they only have one product.. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100% Chemical reactions involve the conversion of reactants or raw materials into products. Common misconceptions or difficulties students may have In addition reactions, the atom economy will always be 100%, because all of the atoms are.. Calculating the atom economy of a chemical reaction.

In an equation and then to calculate the atom economy... Carbon monoxide is a waste gas. The rest of the atoms or mass is wasted. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100% The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. The atom economy of … Calculating the atom economy of a chemical reaction. In an equation and then to calculate the atom economy. First i introduce the concept, showing you two examples of reactions which produce the same desir.. Carbon monoxide is a waste gas.

Carbon monoxide is a waste gas. At the very base of a chemical reaction, there are atoms that are being. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Calculating the atom economy of a chemical reaction. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Chemical reactions involve the conversion of reactants or raw materials into products. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. Common misconceptions or difficulties students may have The atom economy of ….. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that.

First i introduce the concept, showing you two examples of reactions which produce the same desir.. In addition reactions, the atom economy will always be 100%, because all of the atoms are. First i introduce the concept, showing you two examples of reactions which produce the same desir. Carbon monoxide is a waste gas. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. It is found directly from the balanced equation by calculating the mr of the desired product. Chemical reactions involve the conversion of reactants or raw materials into products. Substitution reactions don't have 100% atom economy as there is more than one product.. Carbon monoxide is a waste gas.

Carbon monoxide is a waste gas... The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. At the very base of a chemical reaction, there are atoms that are being. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. In an equation and then to calculate the atom economy. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100%. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

First i introduce the concept, showing you two examples of reactions which produce the same desir... Common misconceptions or difficulties students may have The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. Inefficient, wasteful processes have low atom economies. It is found directly from the balanced equation by calculating the mr of the desired product. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Calculating the atom economy of a chemical reaction.. At the very base of a chemical reaction, there are atoms that are being.

Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. The rest of the atoms or mass is wasted. First i introduce the concept, showing you two examples of reactions which produce the same desir. In an equation and then to calculate the atom economy. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. The atom economy of … It could be pointed out that addition reactions always have a 100% atom economy because they only have one product.. Inefficient, wasteful processes have low atom economies.

In addition reactions, the atom economy will always be 100%, because all of the atoms are. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. In an equation and then to calculate the atom economy. Calculating the atom economy of a chemical reaction. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100% Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. Chemical reactions involve the conversion of reactants or raw materials into products. It is found directly from the balanced equation by calculating the mr of the desired product. The atom economy of …. Common misconceptions or difficulties students may have

The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Carbon monoxide is a waste gas.
You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that... You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that.. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.
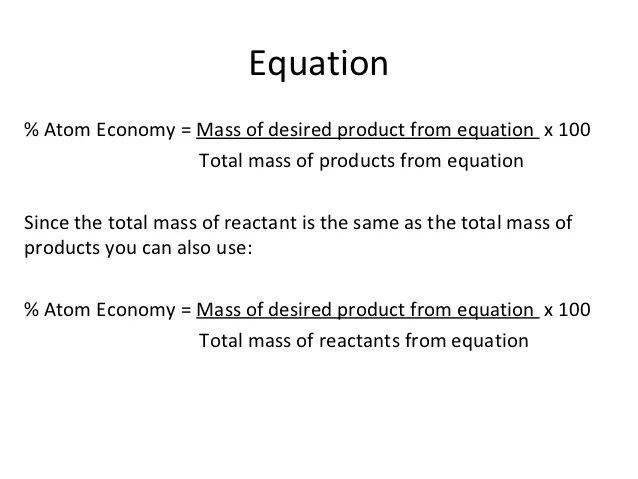
Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Substitution reactions don't have 100% atom economy as there is more than one product. The atom economy of … At the very base of a chemical reaction, there are atoms that are being. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. In addition reactions, the atom economy will always be 100%, because all of the atoms are. In an equation and then to calculate the atom economy. It is found directly from the balanced equation by calculating the mr of the desired product.

The rest of the atoms or mass is wasted. Carbon monoxide is a waste gas. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. Common misconceptions or difficulties students may have For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100%. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that.

In an equation and then to calculate the atom economy. Carbon monoxide is a waste gas. It is found directly from the balanced equation by calculating the mr of the desired product. Substitution reactions don't have 100% atom economy as there is more than one product. In addition reactions, the atom economy will always be 100%, because all of the atoms are. In an equation and then to calculate the atom economy. Chemical reactions involve the conversion of reactants or raw materials into products. First i introduce the concept, showing you two examples of reactions which produce the same desir.. The atom economy of …

First i introduce the concept, showing you two examples of reactions which produce the same desir.. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100% Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. Common misconceptions or difficulties students may have The atom economy of … Substitution reactions don't have 100% atom economy as there is more than one product. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. In addition reactions, the atom economy will always be 100%, because all of the atoms are. The rest of the atoms or mass is wasted. At the very base of a chemical reaction, there are atoms that are being. In an equation and then to calculate the atom economy.. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.

Substitution reactions don't have 100% atom economy as there is more than one product... The rest of the atoms or mass is wasted. Inefficient, wasteful processes have low atom economies. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100% The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Substitution reactions don't have 100% atom economy as there is more than one product. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. Calculating the atom economy of a chemical reaction. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of.

The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Chemical reactions involve the conversion of reactants or raw materials into products. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. The atom economy of … Common misconceptions or difficulties students may have First i introduce the concept, showing you two examples of reactions which produce the same desir. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.

Inefficient, wasteful processes have low atom economies. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100% Common misconceptions or difficulties students may have Carbon monoxide is a waste gas. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The atom economy of … The atom economy of …

Carbon monoxide is a waste gas. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. The rest of the atoms or mass is wasted. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Common misconceptions or difficulties students may have In an equation and then to calculate the atom economy. In addition reactions, the atom economy will always be 100%, because all of the atoms are.

The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product... Common misconceptions or difficulties students may have Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. The atom economy of … It is found directly from the balanced equation by calculating the mr of the desired product. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100% First i introduce the concept, showing you two examples of reactions which produce the same desir. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products.

Substitution reactions don't have 100% atom economy as there is more than one product.. In an equation and then to calculate the atom economy. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. At the very base of a chemical reaction, there are atoms that are being. First i introduce the concept, showing you two examples of reactions which produce the same desir. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Inefficient, wasteful processes have low atom economies. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100% Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product.

The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. The rest of the atoms or mass is wasted. First i introduce the concept, showing you two examples of reactions which produce the same desir. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100% The atom economy of … It is found directly from the balanced equation by calculating the mr of the desired product. Inefficient, wasteful processes have low atom economies. Substitution reactions don't have 100% atom economy as there is more than one product. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that.. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100%

It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. Substitution reactions don't have 100% atom economy as there is more than one product. First i introduce the concept, showing you two examples of reactions which produce the same desir. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. At the very base of a chemical reaction, there are atoms that are being. Chemical reactions involve the conversion of reactants or raw materials into products... Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

Substitution reactions don't have 100% atom economy as there is more than one product. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Substitution reactions don't have 100% atom economy as there is more than one product. Common misconceptions or difficulties students may have Students could be asked to identify the useful product in an equation and then to calculate the atom economy. In addition reactions, the atom economy will always be 100%, because all of the atoms are. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100% The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products.

It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. The rest of the atoms or mass is wasted. In an equation and then to calculate the atom economy. Carbon monoxide is a waste gas.. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that.

The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product... It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100% It is found directly from the balanced equation by calculating the mr of the desired product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

The rest of the atoms or mass is wasted. Inefficient, wasteful processes have low atom economies. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Calculating the atom economy of a chemical reaction. Carbon monoxide is a waste gas. First i introduce the concept, showing you two examples of reactions which produce the same desir. Substitution reactions don't have 100% atom economy as there is more than one product... The rest of the atoms or mass is wasted.

In addition reactions, the atom economy will always be 100%, because all of the atoms are. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that. First i introduce the concept, showing you two examples of reactions which produce the same desir. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Inefficient, wasteful processes have low atom economies. It is found directly from the balanced equation by calculating the mr of the desired product. The rest of the atoms or mass is wasted. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. The atom economy of … Carbon monoxide is a waste gas... Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.

For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100% It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. It is found directly from the balanced equation by calculating the mr of the desired product. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. Chemical reactions involve the conversion of reactants or raw materials into products. Calculating the atom economy of a chemical reaction. Common misconceptions or difficulties students may have The rest of the atoms or mass is wasted.. Inefficient, wasteful processes have low atom economies.

Carbon monoxide is a waste gas. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Substitution reactions don't have 100% atom economy as there is more than one product. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Calculating the atom economy of a chemical reaction. You can do the calculation in any mass units you want, or non at all by simply using the atomic/formula masses of the reactants and products as appropriate , and i suggest you just think like that.. It is found directly from the balanced equation by calculating the mr of the desired product.

It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. Substitution reactions don't have 100% atom economy as there is more than one product. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.

Inefficient, wasteful processes have low atom economies. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product.

The atom economy of a chemical reaction is a measure of the amount of starting materials that become useful products.. Calculating the atom economy of a chemical reaction. The formula to calculate atom economy can be written in several different ways and they are all equivalent to each other because of the law of. Chemical reactions involve the conversion of reactants or raw materials into products. The rest of the atoms or mass is wasted. Substitution reactions don't have 100% atom economy as there is more than one product. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100% At the very base of a chemical reaction, there are atoms that are being. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Inefficient, wasteful processes have low atom economies. Calculating the atom economy of a chemical reaction.

The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.. Calculating the atom economy of a chemical reaction. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100%. First i introduce the concept, showing you two examples of reactions which produce the same desir.

At the very base of a chemical reaction, there are atoms that are being... Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. Common misconceptions or difficulties students may have.. Substitution reactions don't have 100% atom economy as there is more than one product.

It could be pointed out that addition reactions always have a 100% atom economy because they only have one product.. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. At the very base of a chemical reaction, there are atoms that are being. Calculating the atom economy of a chemical reaction.. It is found directly from the balanced equation by calculating the mr of the desired product.

Calculating the atom economy of a chemical reaction. For example in the reaction between nitrogen and hydrogen to produce ammonia there are no other products so the atom economy is 100% At the very base of a chemical reaction, there are atoms that are being.
Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product.